Tired of Taking Blood Thinners?
Join us for a free monthly webinar about two procedures to reduce stroke risks.
Preventing stroke is one of the most important aspects of atrial fibrillation (AFib) care at Valley. While many patients take blood thinners to lower their risk, Valley also offers the Watchman device for certain patients.
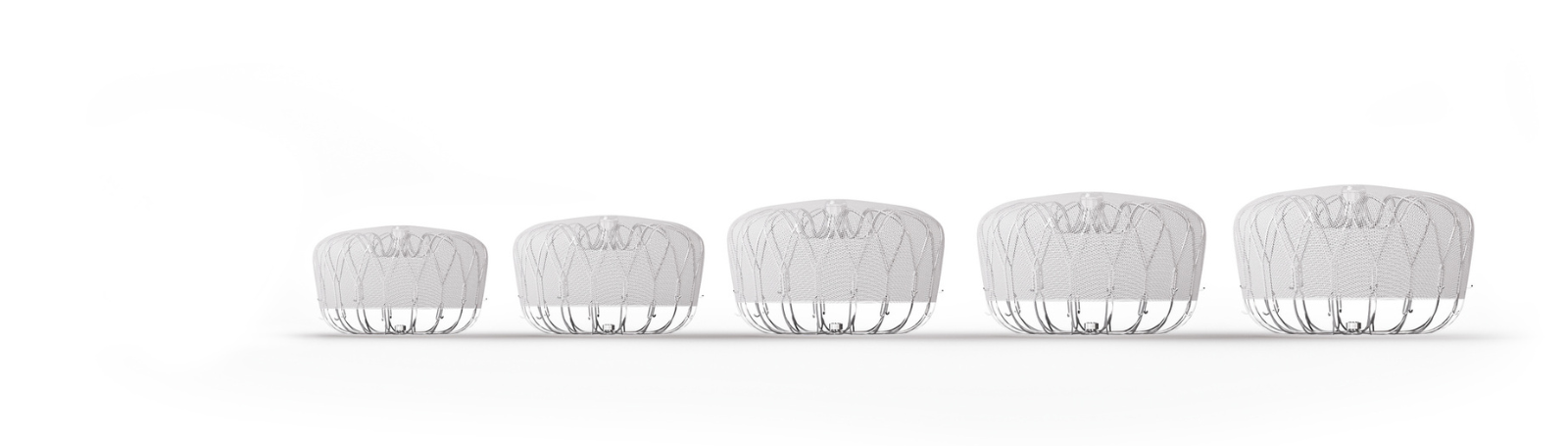
The Watchman is an implant that helps prevent stroke in people who have AFib not caused by heart valve problems. This can be an alternative to lifelong use of blood thinner medicines for certain people.
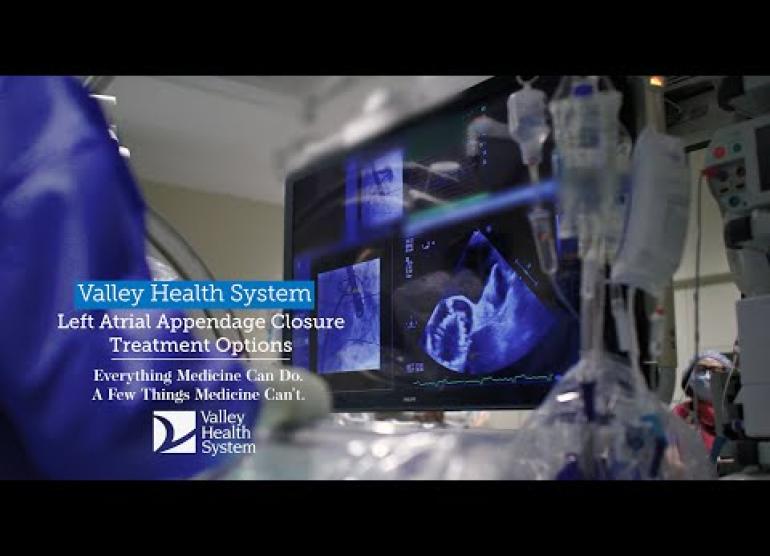
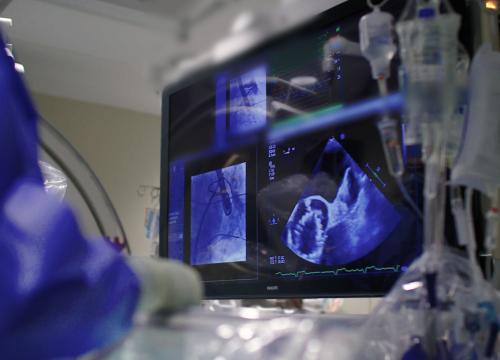
Valley’s electrophysiology team has vast experience in left atrial appendage (LAA) closure procedures with the Watchman device. The team is quickly approaching their 600th minimally invasive procedure using the Watchman device to achieve high-quality outcomes.
At Valley, we offer the latest version of the device, the Watchman FLX Pro. Research shows this device has even better outcomes than the previous version. It also allows a wider range of people to reduce their stroke risks without blood thinners.
Am I a Candidate for the Watchman?
The Watchman device might be right for you if you have AFib that is not caused by heart valve disease. It could be a good option for you if you have one of the following:
- A history of major bleeding while taking blood thinners
- A career or lifestyle that increases the risk for major bleeding due to trauma
- Difficulty controlling blood clots with blood thinners
Talk to our electrophysiology team at Valley to find out if the Watchman may be right for you. If not, you may be a candidate for another minimally invasive procedure with the Amulet device.
How Does the Watchman Work?
AFib affects how your heart contracts, which leads to blood pooling in a pouch called the left atrial appendage (LAA). The LAA is where most blood clots form. If blood clots dislodge and travel through your bloodstream to the brain, it can lead to a stroke.
The Watchman implant is a tiny mesh umbrella that closes the LAA and keeps blood clots from entering the bloodstream. This reduces your risk of stroke.
Many people with AFib take blood thinners, like warfarin, to prevent stroke. With the Watchman device, you may be able to stop taking these medications.
Preparing for Your Watchman Procedure
Here’s what you can expect if you are preparing for a Watchman procedure:
- You will come to Valley for a computed tomography (CT) scan using advanced software called TruPlan. Our team uses this software to plan your procedure, which ultimately results in less time under anesthesia and fewer complications.
- We will plan where to implant the Watchman from your CT scan. We will also choose the right size of the device to use during your procedure.
- Our team will give you information about what to bring to the hospital before your procedure. We’ll also give you instructions for eating, drinking and taking your medications beforehand.
What to Expect During Your Watchman Procedure
Here’s what you can expect if you have a Watchman procedure:
- Plan to have someone drive you to and from the hospital on the day of your procedure. You’ll need someone to drive you home after the procedure.
- At the hospital, an anesthesiologist will give you general anesthesia to put you to sleep.
- Your electrophysiologist and interventional cardiologist will make a small incision in your groin to reach a vein.
- They will insert a catheter (thin tube) with the Watchman device into the vein. Using advanced image guidance, we will direct the catheter through the heart to the LAA.
- They will place the Watchman over the LAA opening and then remove the catheter.
- They will close the incision with a suture (stitch).
- The procedure takes about an hour.
Recovery After a Watchman Procedure
Here’s what you can expect after a Watchman procedure:
- Most people stay overnight in the hospital and go home the following day. Some people can go home the same day.
- Recovery time is minimal. You should be able to return to your normal activities in a few days.
- You will still need to take blood thinners for a few months after your procedure. Your doctor will discuss the best regimen for you.
Why Choose Valley for the Watchman Procedure?
- Advanced expertise in LAA closures: Our electrophysiologists and interventional cardiologists have advanced expertise in left atrial appendage closures. We offer the full range of treatment options, including the Watchman FLX Pro and the Amulet device. We’ll work closely with you to determine the best option for your specific needs.
- Comprehensive AFib care: Valley’s Snyder Center for Atrial Fibrillation brings together a team of specialists to treat your AFib. They provide everything from medication management and nutrition counseling to ablation and implants. They’ll also address other conditions often associated with AFib, like high blood pressure, sleep apnea, obesity and stress.
- Access to clinical trials: Valley offers clinical trials related to AFib and other heart rhythm conditions. This gives you access to treatments and approaches that aren’t widely available.
- National leaders in electrophysiology: Our electrophysiologists are actively involved in the Heart Rhythm Society and publish many important studies each year. As leaders in the field, they’ll offer you the most up-to-date treatments to address your specific heart rhythm condition. This offers you the highest quality care within your own community.