April 6, 2021
Valley is Now Enrolling Patients in Clinical Trial to Help Advance Cardiovascular Care
Cardiac electrophysiologists at The Valley Hospital are the first in the New York Tristate area (New Jersey, New York, and Connecticut) to test a new type of ablation technology that uses pulsed electric fields to treat patients with atrial fibrillation (AFib).
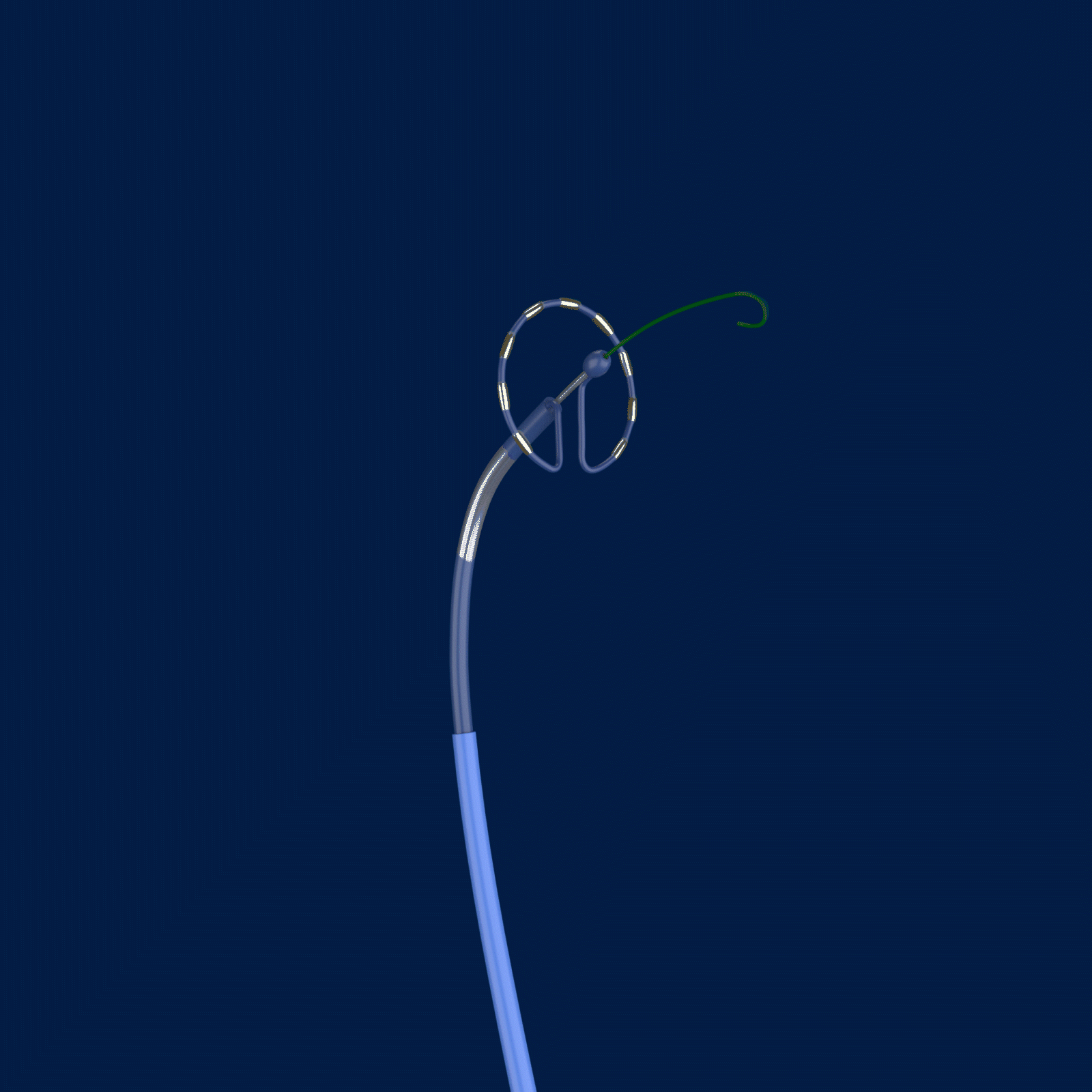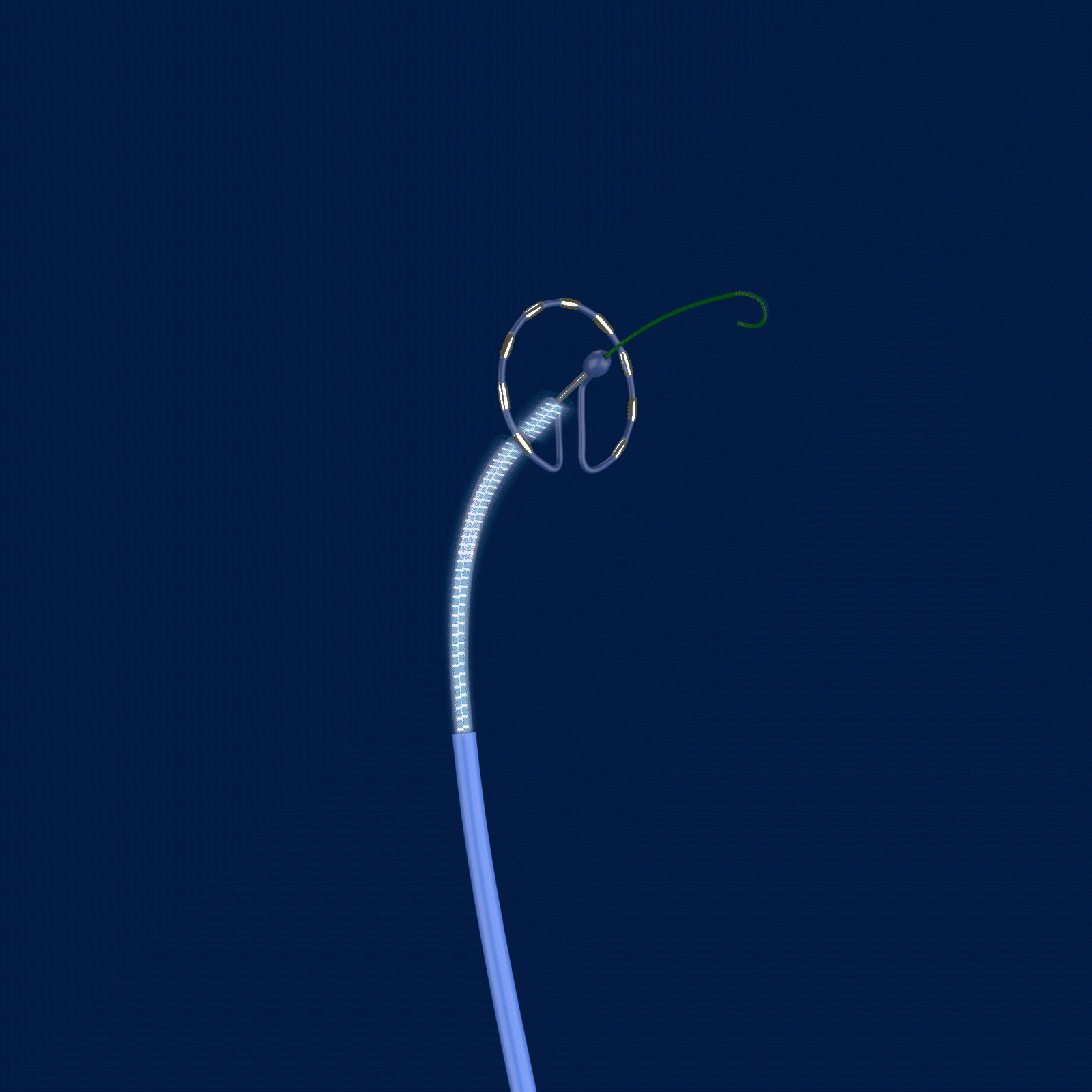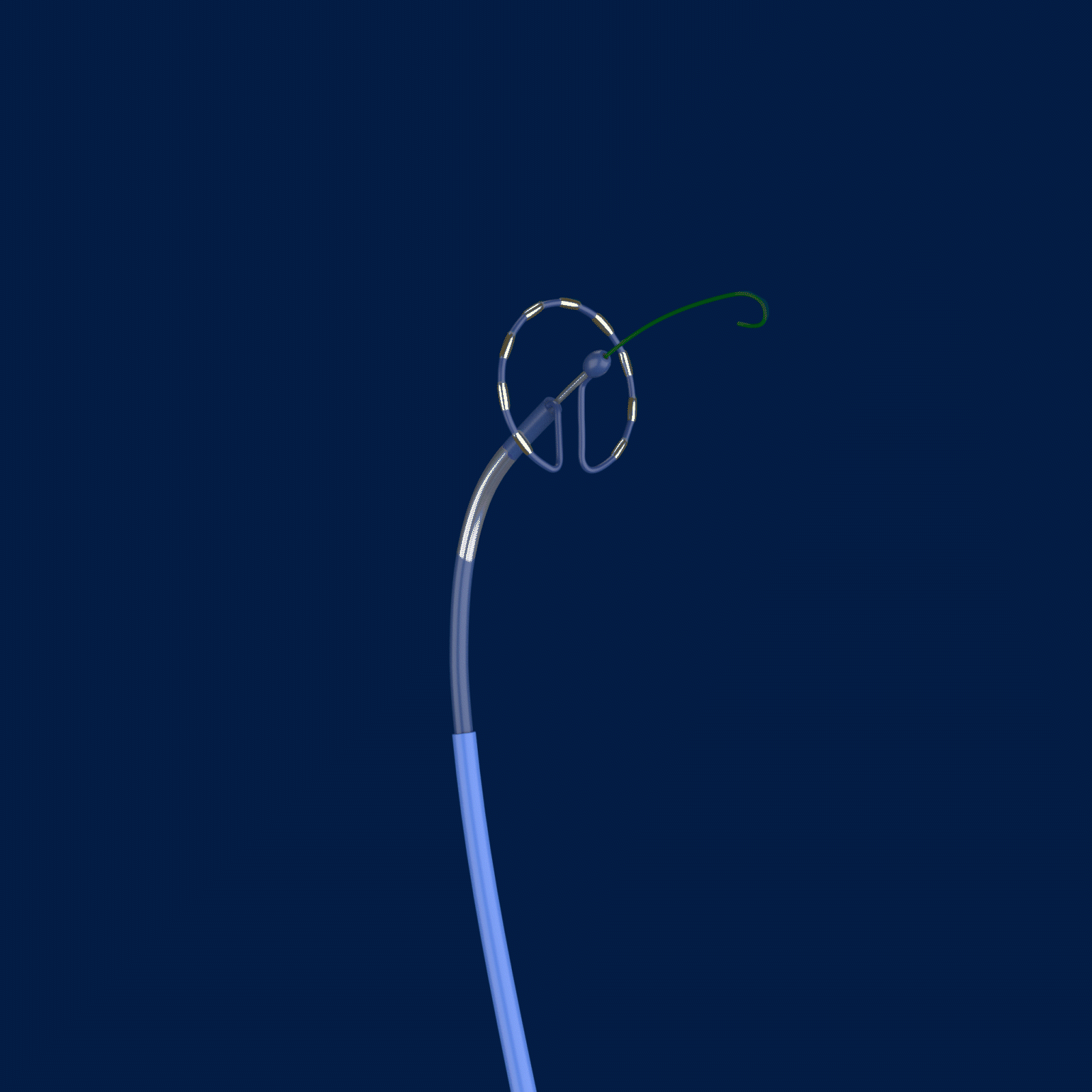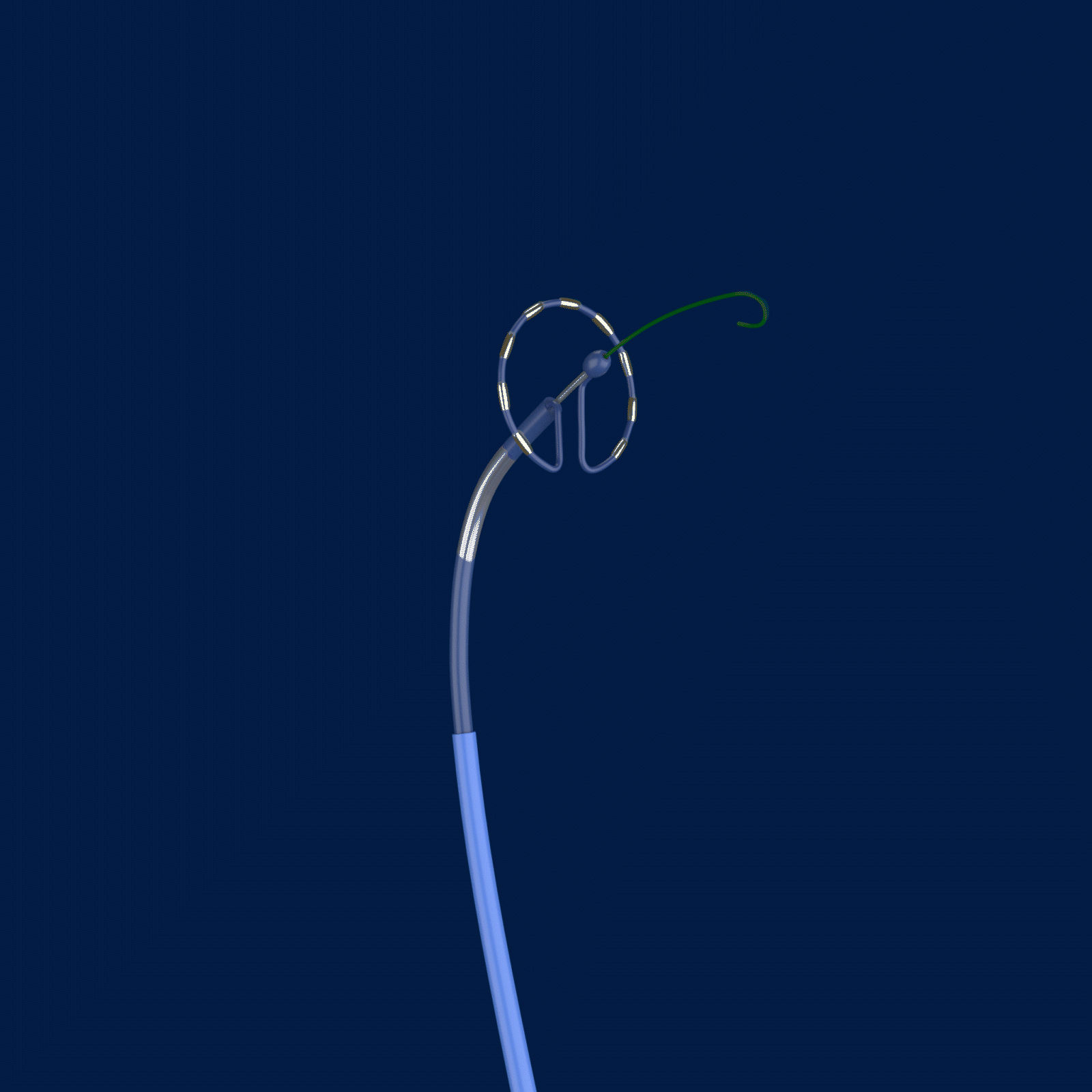
The trial is called PULSED AF, and it uses the Medtronic PulseSelect™ Pulsed Field Ablation (PFA) System to treat paroxysmal or persistent AFib by interrupting irregular heart rhythms.
AFib impacts more than 37 million people worldwide and is one of the most common cardiac arrhythmias. In patients with AFib, the upper chambers of the heart — or atria — quiver or beat very fast and irregularly, so the heart cannot effectively pump blood to the rest of the body.
Ablation, a common treatment option for those with AFib, targets the cause of irregular heart rhythms by creating scar tissue and lesions. The PulseSelect PFA System delivers pulsed electric fields through an ablation catheter designed specifically to interrupt irregular electrical pathways in the heart that trigger atrial fibrillation. However, unlike traditional methods of ablation that heat (radiofrequency ablation) or cool (cryoablation) the atrial tissue, the PulseSelect PFA System uses a non-thermal approach called irreversible electroporation and preferentially targets heart tissue with the goal of avoiding unwanted injury to surrounding tissues.
“We are always looking for innovative ways to provide the highest quality of care possible to our patients,” said Dr. Suneet Mittal, Director of Electrophysiology and Medical Director of the Snyder Center for Comprehensive Atrial Fibrillation at The Valley Hospital. “This technology appears to eliminate one of the risks associated with traditional catheter ablation — damage to surrounding tissue. If confirmed in the clinical trial, this has the potential to be a huge advance in the field of atrial fibrillation ablation.
“Valley has a robust cardiac clinical trials program, and we are proud to play a leading role in participating in trials that help advance the treatment of patients with atrial fibrillation and other cardiac conditions,” Dr. Mittal added.
About PULSED AF
The PULSED AF trial is a prospective, non-randomized, multi-center clinical trial that will enroll up to 500 patients who will be treated with the PulseSelect System across as many as 50 sites in the United States, Canada, Europe, and Australia. PULSED AFib is designed to evaluate the safety and efficacy of the PulseSelect PFA System for the treatment of AFib in adult patients with a history of drug-refractory, recurrent and symptomatic paroxysmal or persistent AF. Patients will be assessed at six and 12 months.
Caption: Cardiac electrophysiologists at The Valley Hospital are the first in the New York Tristate area (New Jersey, New York, and Connecticut) to test a new type of ablation technology for patients with atrial fibrillation. The PulseSelect PFA System delivers pulsed electric fields through an ablation catheter designed specifically to interrupt irregular electrical pathways in the heart that trigger atrial fibrillation, while avoiding damage to surrounding tissues.

